Interested in Instrumentation?
Get Instrumentation articles, news and videos right in your inbox! Sign up now.
Instrumentation + Get AlertsBecause of the high solubility of both caustic soda and lime, pH often becomes biologically prohibitive before ideal alkalinity levels and process stability can be reached.
Added without proper feed controls, the use of lime in, or prior to, primary treatment can actually reduce alkalinity going into the secondary treatment processes by precipitating CaCO3 in the primary clarifiers.
The EPA Wastewater Technology Fact Sheet: Chemical Precipitation EPA 832-F-00-018 states:
- Lime produces calcium carbonate in wastewater, which acts as a coagulant for hardness and particulate matter.
- Lime is an effective phosphate removal agent, but results in a large sludge volume.
- By itself, large quantities of lime are required for effectiveness.
- Lime typically generates more sludge than other coagulants.
- The addition of lime may increase the volume of waste sludge up to 50 percent.
Thioguard vs. lime slurry and caustic soda
Thioguard’s customers have stated that they are being told lime slurry is similar to Thioguard (municipal-grade magnesium hydroxide), and can be used as an alternative in the many applications in which Thioguard is used. The simple fact: Thioguard, lime slurry and caustic soda all have different physical and chemical properties that affect how each responds to, and reacts with, the systems to which they are added. And, because of these differences in properties, the impacts they impart and the utility for their use are starkly different.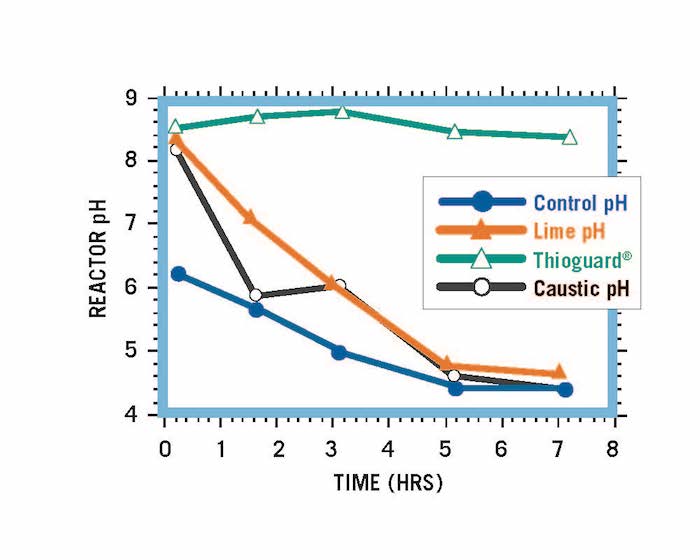
Reactor systems treated to an initial pH of 8.5 using Thioguard, caustic soda and lime. Equivalent amounts of acid added to each over time.
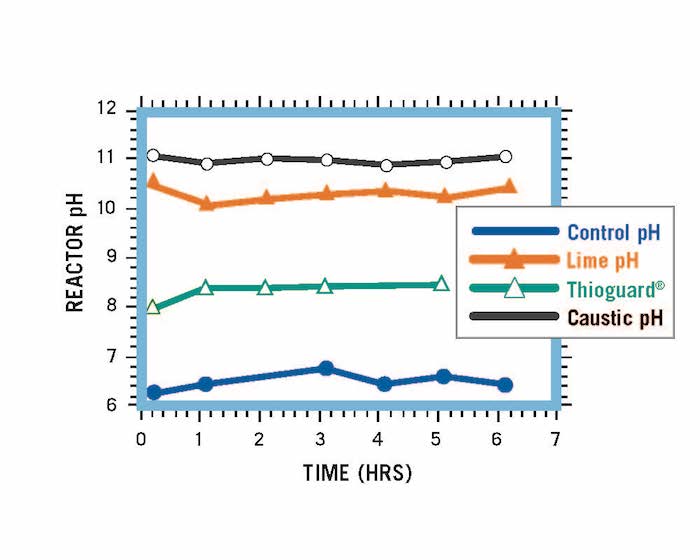
Reactor systems treated with an equivalent amount of Thioguard, caustic soda and lime. At the elevated pHs for caustic soda and lime, solids and scale begin to form and cause problems.
Miles of piles
The use of lime generates significant amounts of sludge in wastewater collections and treatment. On a chemical basis, one ton of lime can generate as much as 11.5 to 15.5 tons of 20 percent sludge cake to remove or dispose. In contrast, Thioguard reactions in wastewater produce only water and water-soluble products as TDS with no added sludge. In fact, customers using Thioguard have reported reductions of 15 to 25 percent in total solids/sludge produced, due to a combination of improved biological performance and reduced inorganic solids loading. To see more, go to www.thioguard.com, click Downloads, and view “Thioguard Takes the Cake and Makes it Better” and “Thioguard Bridges the Cation Gap – Saves Biosolids $$.”
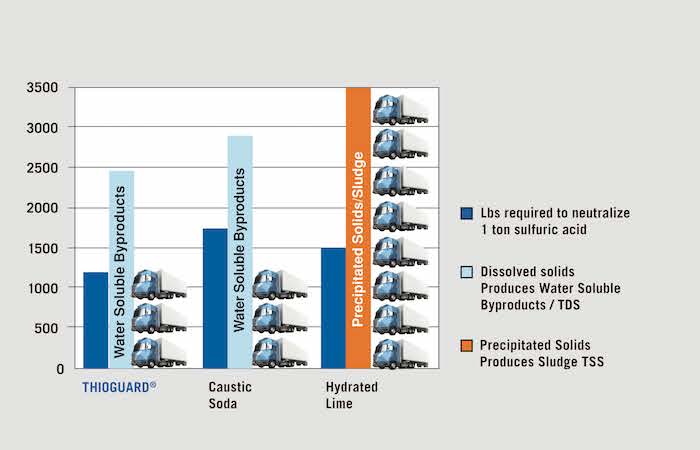
Hydrated lime added to collections systems increases O&M costs related to formation of scale and accumulated solids/sludge. In severe cases, it leads to line blockages and SSOs.
Unexpected consequences
It is often difficult to balance the competing reactions of lime softening and alkalinity supplementation with the use of lime in wastewater.
With growing needs for alkalinity in nitrification and BNR processes, operators are often faced with the challenge of supplying higher levels (in excess of 30 mg/L) of alkalinity without upsetting processes or depleting the chemical budget.
On the surface, the cheap cost of lime is an appealing choice. When evaluated in its entirety, it is rare that lime is the most cost-effective choice for pH modification and alkalinity supplementation.
Why? Lime softening often gets in the way of alkalinity supplementation. Through unintended lime softening, alkalinity levels can be depleted going into secondary treatment. This alkalinity depletion often goes unnoticed because it is masked by pH elevation, which is often assumed to be linked proportionately to alkalinity.
Moreover, unless pH is carefully monitored at both bioreactor influent and effluent, pH spikes and instability in the aeration basins and BNRs leads to inefficient biological processes. This inefficiency prompts many operators to increase MLSS, MCRTs/SRTs, and oxygen addition, which in turn leads to increased energy and solids disposal costs.
Thioguard’s specific chemical and physical properties add alkalinity to the water system, moderately modify pH, stimulate biological activity and improve solids quality.
Imitation is the sincerest form of flattery
For years, competitors have claimed that Thioguard’s odor control strategy would either not work at all or would lead to downstream flare off of H2S, but academic and field studies have confirmed the superior performance of Thioguard’s unique chemical and physical properties in reducing H2S and other organic odors and improving performance of wastewater treatment plants. In recent years, some competitors have tried to duplicate the results using caustic soda or lime, ignoring that these hydroxides lack the physicochemical properties of municipal-grade magnesium hydroxide ... but the efforts only help validate the successes of Thioguard.
For more information, visit www.thioguard.com.






